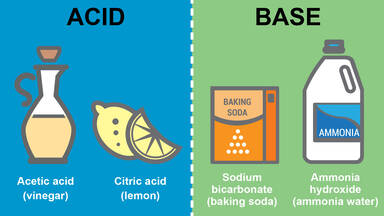
Acids And Bases worksheets
Access printable acids-and-bases worksheets
Acids are __________, which means they "eat away" at other materials.
transitional metal
alkaline
flammable
corrosive
Acidic solutions exhibit a higher ratio of H3O+ ions, known as...
neutrons
hydroxide
electrons
hydronium
When you place blue litmus paper into an unknown clear solution, the blue litmus paper turns red.
Which of the following represents the most logical conclusion?
The substance has a pH value of "2".
The substance is a base.
The substance has a pH value of "7".
The substance is acidic.
What range of values on the pH scale represent solutions with a higher ratio of hydroxide (OH-) ions?
below 7
7
above 7
0-14
Do acids and bases conduct electricity?
Only acids conduct electricity
Only bases conduct electricity
Bases and acids conduct electricity
Neither conduct electricity
Identify the gas evolved.
Hydrogen
Nitrogen
Carbon dioxide
Oxygen
Which one of the following can be used as an acid-base indicator by a visually impaired student?
Litmus
Turmeric
Methyl Orange
Vanilla essence
Which of the following salts does not contain water of crystallisation?
Blue vitreol
Baking soda
Washing soda
Gypsum
Which of the following salts will give an aqueous solution having pH of almost 7?
Ammonium nitrate
Ammonium chloride
Calcium chloride
Sodium chloride
What is a titration?
when the moles of hydrogen ions is equal to the moles of hydroxide ions
adding a known amount of solution of known concentration to determine the concentration of an unknown
reaction in which an acid and a base react in an aqueous solution to produce a salt and water
the extent of ionization of an acid or base
Zinc granules on treating with an acid X, forms zinc sulphate (ZnSO4) salt along with the evolution of a gas Y which burns with a pop sound when brought near to a burning candle. Identify the acid X and gas evolved Y.
X-Sulphuric acid and Y- Oxygen gas
X- Hydrochloric acid and Y- Oxygen gas
X- Sulphuric acid and Y- Hydrogen gas
X- Hydrochloric acid and Y- Hydrogen ga
Which of the following indicators turn red in an acidic solution?
phenolphthalein
red litmus
turmeric
methyl orange
Identify the products of the following reaction:
CaCO3 + 2HCl -->
Calcium hydrogen carbonate and chlorine gas
Calcium chloride and water
Calcium oxide, carbon dioxide and water
Calcium chloride, carbon dioxide and water
Other topics to explore
Do more than worksheets with Quizizz
Interactive acids-and-bases worksheets & quizzes
Quizizz is an online platform that helps teachers create interactive worksheets for their students to learn science, specifically chemistry. Teachers can create quizzes on acids and bases to help students understand the concepts better and assess their knowledge. Quizizz is an easy and fun way to engage students in learning chemistry and other science topics.